|
Did you know? One of those nutrients, nitrogen, comes in many forms, such as ammonia, ammonium, urea and nitrates. It serves as food for living contaminants in water. But it also combines with chlorine in some very complex chemical reactions with multiple stages, requiring more and more chlorine to eradicate it, in the process of breakpoint chlorination. The nitrogen compounds directly increase chlorine demand. that many believe chlorine reacts to and destroys phosphates directly, leading to increased chlorine demand — even we were taught that for years! But that’s not the case. In fact, chlorine and phosphate don’t even interact in the water. generation after generation of spawning algae. You had plenty of killing power with your chlorine (and perhaps an algaecide too), but because the generations kept coming back, the chlorine eventually got used up. You never removed the nutrients. Unlike nitrogen compounds, phosphates can be easily removed, but this is done through the use of phosphate removers, not chlorine. So if you’re facing a high chlorine demand, direct sunlight may not be the culprit. Perhaps it’s oxidation of bather waste or nitrogen compounds; or the repeated sanitization of rapidly reproducing microorganisms such as algae. We recommend supplementing chlorine accordingly, because we are relying on it to do many jobs." Excerpt from Pool & Spa News https://www.poolspanews.com/how-to/maintenance/the-finer-points-of-water-treatment_o
0 Comments
Spring is about to, er...Spring. Here are some Spring Cleaning tips. Safety Inspection This is unarguably the most important pre-Spring chore, one that cannot be overlooked.
The pool must not be used until all broken or damaged equipment is replaced or repaired.Pool Water Chemistry It's a good idea to start off the season with a well-balanced, protected pool.
Have you been thinking of an upgrade?
If your single-speed pump has been screeching all winter, it may be already too late to either replace the motor or the entire pump. The U.S. Department of Energy has banned the purchase or sale of all single and dual-speed pool pumps and motors greater than 1 THP (True Horsepower). But it's not too late to upgrade to a DOE-compliant Variable Speed (VS) Pump! These pumps currently qualify for consumer rebates through your local electric utility. But when those money-saving VS pumps become the norm, those rebates will get smaller and smaller. The cost of chlorine is on the rise! Now is the time to look at the new technologies available that will significantly reduce the use of chlorine. Check these out:
There aren’t many moving parts on your filter and no electricity, but one thing that is present in every filter is pressure, and it is that pressure that drives the whole mechanism to carry on with its mission of cleaning your water. That is why you will find a little handy pressure gauge on the top of every filter. With it, you can read the pressure on your filter and use that number as a guide to properly care for your pool. What Is Your Pressure Gauge? The pressure gauge is a simply little dial that is usually found on the top of your pool’s filter system. Most include ranges from 0 to 60 pounds per square inch (psi) that indicate how the pressure is holding (or not holding) in your pool. Unfortunately, this number is relative. Ideally, it is the number that you record when you first install your pool filter and everything in your pool is running just as it should. If you don’t have that number, then your next best result is directly after you have changed the filter or, at the very least, given it a good cleaning. Whichever route you have to take, make sure you record that number somewhere for future reference. Now that you have the number, you need to make sure you start checking the pressure at least once every week as a part of your normal pool maintenance routine.
problems that indicate you have a problem with your pool. However, sometimes it can be a problem with the pressure gauge. These gauges don’t last forever and usually cost less than $20 to replace, so you could actually have a problem with your gauge and not your pool.
If your gauge has some age on it or it is cracked, you may want to consider investing $10 to check to make sure you really do have a pressure problem and not just a gauge problem. In the end, it could save you quite a bit of money. While the pressure gauge is just a small component in a large system, its role is vital to the overall health of your pool. Remember to check it weekly and never ignore pressure problems of any kind. If you do, you could end up doing more damage to your pool resulting in much more expensive repairs down the road. The change of seasons has brought shorter days and cooler nights. The cool weather can make small pool problems more apparent. Now is the time to deal with these issues before they become a major repair expense. Cause: Air leak on the suction side of the pump. Air pulled into the pump causes it to lose prime, but only temporarily. This intermittent loss of prime is making the pump cavitate, creating the strange pump noise. Other symptoms can be:
Rather than going through a lengthy list of likely causes, let's zero in on the least expensive, most accessible part of the pump, the strainer cover (pump lid) O-ring. The strainer cover o-ring makes the seal between the cover and the pump housing. Without it, the pump will never prime. A flattened, stiff, or cracked o-ring will sometimes function normally. The problem will pop-up when the pump is required to work a little harder, like:
To prevent this problem from popping up at the most inconvenient time, inspect the o-ring every time you clean the pump basket.
the pump will not come on while you are gone!) and/or a picture of the pump itself.
Next, clean the pump cover and sealing surface of the pump housing with warm soapy water. Apply a film of o-ring lubricant (non-petroleum) to the new o-ring, fill the pump housing with water, reassemble the pump and turn it on. Make sure the pump stays primed and the water flow is back to normal. Chemical Fire Raises Concerns about Trichlor Supply The blaze at a BioLab plant occurred shortly after it was struck by Hurricane Laura. .By Rebecca Robledo Published in Pool & Spa News 09/22/20 No injuries or illnesses were reported as a result of the fire. According to BioLab parent company KIK Custom Products, the facility had already been evacuated to make way for the storm, and all employees were confirmed safe. The State Police Hazmat Unit is conducting an investigation on the cause and to assess damages. It likely will take several months, said Sargent James Anderson of the State Police. After seeing the site, the department suspects that the incident was caused when water entered an enclosed area that had been breached because of storm damage. However, this is a preliminary theory, and it won’t be finalized until the investigation is complete. BioLab and KIK are still assessing the extent of damage and lost product, said Isabelle Pierre, general counsel for KIK Custom Products. It is known what chemical was involved — trichloroisocyanuric acid. This leaves some industry professionals worrying about the availability of trichlor for the season. More than usual of the ever-popular sanitizer may be needed this year, as children continue schooling at home and families extend their swim seasons. Additionally, professionals are receiving notices from manufacturers about price increases. This includes a 7½% rise in the price of liquid chlorine, causing even more concern about the effects of the BioLab fire, said David Hawes, CEO of H&H Pool Services in Dublin, Calif., and CFO of the Independent Pool and Spa Service Association. “We’re all sitting on pins and needles to see if the [fire] will affect chlorine tablet supply and pricing,” he said. To make sure his company is set for a protracted swim season, he is stocking up. “It seems weird, because normally in September you’re starting to slow down a little bit. But you can store some of the dry chemical without too much trouble, so we’re bulking up a little bit. If the price doesn’t go down, I’ll just be stocked up.” How does this impact you, a Pool Owner? Before the fire, we were finding it difficult to find some trichlor products, mostly 1" Chlorine Tabs. Now, stock at distribution warehouses have been depleted and we do not have a clear timetable of when supply will return to normal. If you use trichlor tablets, Pacific Pool Supply has alternatives for you! poolife® NST® Tablets (for skimmer use)NST® is an alternative to 3" tablets and does not contain cyanuric acid. Perfect for use in a skimmer, this product will destroy bacteria and organic contaminants without over-stabilizing your pool water or adding additional cyanuric acid to your water. Each long-lasting tablet will continuously sanitize your pool in the skimmer for up to one week! This product is an innovative, proprietary technology that provides long-lasting, slow-dissolving chlorination sanitization levels. Useful Tips:
poolife® NST® Feeder Tablets
These slow-dissolve cal hypo tablets were specifically formulated for use in the poolife® NST® Tablet Feeder. They contain a stain and scale inhibitor and are packaged in modular pails for easy identification at the shelf. Just like the NST® skimmer-fed tablets, these will destroy bacteria and organic contaminants without over stabilizing the pool or adding additional cyanuric acid. Useful Tips:
As recently published by HASA’s Terry Arko in Aqua Magazine Defining what liquid sodium hypochlorite is. In the swimming pool industry, one of the most popularly chosen forms for sanitizing water is liquid sodium hypochlorite. Commonly known as liquid chlorine it is widely used in swimming pools both commercial and residential. It is a bit of a misnomer to use the term liquid chlorine. Technically speaking it can better be described as a chlorinating compound. It would more correctly be referred to as liquid bleach. Sodium hypochlorite is made up of a blend of liquid chlorine, water, and sodium hydroxide.
A brief history of liquid sodium hypochlorite, its other uses, etc. The discovery of using chlorine for bleaching goes as far back as 1787 when a French chemist named Claude Berthollet developed a weak sodium hypochlorite solution by passing chlorine gas through sodium carbonate (soda ash). The product he created was known as Eau De Javelle or Javel Water. The name came from the region near Paris where it was manufactured. In 1820 Antoine Labarraque discovered that using sodium hydroxide with chlorine gas sodium hypochlorite could be produced much cheaper than with soda ash. Labarraque discovered many of the disinfectant properties of liquid sodium hypochlorite and is largely responsible for promoting its use worldwide.
History of using public pools to backyard pools Since the “hypochlorites” had proven effective in the disinfection of waterborne diseases in drinking water, it was only natural that chlorination would make its way to swimming pools. Both calcium hypochlorite and liquid sodium hypochlorite had been the two main treatments available other than chlorine gas. In particular, liquid sodium hypochlorite was preferred at public pools because it was safer to store than either chlorine gas or calcium hypochlorite. As pools began to grow in popularity beginning in the 1920s the use of sodium hypochlorite found a new and growing industry. In the mid-1940s the polio panic had people afraid to go to public pools. Liquid sodium hypochlorite became very popular at this time as a disinfectant because of chlorine’s proven ability to kill polio. Public pools stocked up and chlorinated pools in order to win back the fearful public.
Sanitize with liquid sodium hypochlorite and manage pH with muriatic acid. Moving into the 1970s a newer chlorine compound was beginning to show up in backyard swimming pools. The new kid in town was the stabilized chlorine tablet known as Tri-Chlor. How tablet convenience replaced liquid practicality in the market While calcium hypochlorite and liquid sodium hypochlorite were enjoying mainstream popularity in backyard pools there was a problem. That problem was the fact that both forms of hypochlorite were un-stabilized. That meant that in the summer sun the free chlorine created by these un-stabilized compounds didn’t last very long. In fact, nearly all the chlorine from liquid sodium hypochlorite could be destroyed in about four hours by the direct summer sunlight. Since this problem began to be recognized the practice of adding extra amounts of liquid sodium hypochlorite in the late afternoon or evening was incorporated. In 1956 Monsanto began to produce and distribute cyanuric acid (CYA). At levels of 30 ppm, CYA helped free chlorine residuals last up to eight times longer than without the additive. There was incredible benefit from adding CYA to the pool water. In the late 1960’s and early 1970’s solid tablets of stabilized chlorine known as isocyanurates began to make their way into residential swimming pools. The selling point was a convenience for the owner. The tablets could be added to an inline feeder or a floating container known as a floater. The advantage was that the stabilizer to make chlorine last was built right into the tablet. Voilà, instant convenience for the modern pool owner. As this system of treating backyard pools grew in popularity liquid sodium hypochlorite was pushed out as the main source of pool sanitization. The liquid was now mainly used as a backup or shock to Tri-Chlor tablets in pools.
This was known as “chlorine lock” and whether it was mythical or factual it was the source of many a barroom brawl. From academia to the field tech the debate over CYA has been a mainstay in the swimming pool industry for over 30 years. One thing that began to become certain on an anecdotal basis was that pools that used Tri-Chlor tabs with increasing amounts of CYA were becoming a struggle to maintain. It seemed more of a challenge to keep free available chlorine levels and many pools had algae problems toward the end of the season. Users of trichlor tabs began to become aware of some side effects from use that led to complications in water treatment. What mainly began to be revealed was the amount of CYA being released from the continual dependence on Tri-Chlor tablets as the primary means of chlorination. Most of an 8 oz. Tri-Chlor tablets consist of CYA. Over 54% by weight is CYA with the other 46% being chlorine. So, it was realized that a lot of CYA comes from Tri-Chlor tabs. However, there was a large contingent of folks under the impression that more is better, while others were beginning to see the need to drain and dilute to keep levels down. Regardless of the stance on the CYA debate, one point was clear. Tri-Chlor tabs made the maintenance of pool water more complicated. Some other points regarding this were the fact that Trichlor tabs were very acidic which leads to using more soda ash to balance pool water. Then the discovery of how CYA interferes with the total alkalinity reading if the levels are higher than 60 ppm. Another adjustment to be made. Next came emerging research from the Centers for Disease Control (CDC) pointing out that in the cases of crypto remediation it takes a lot more chlorine and contact time to inactivate the parasite if CYA levels are present even as low as 15 ppm.Dealing with bacteria and preventing algae reports have come out showing there must be a proper ratio between the ppm of free chlorine and CYA present in the pool. Some suggest using a ratio of 7.5% ppm of free chlorine times the CYA ppm-level. For example, if the CYA level is 60 ppm it would take 4.5 ppm of residual chlorine to kill bacteria and prevent algae growth. 60 x 7.5%=4.5 ppm. The latest report from the CDC and Model Aquatic Health Code (MAHC) ad hoc committee on CYA suggest a CYA to chlorine ratio of 20:1 in order to effectively inactivate bacteria. That means at 20 ppm CYA there must be 1 ppm of FC in order to effectively inactivate bacteria such as E. coli or Giardia. While the debate on all of this continues it is clear to see that the perceived convenience of Tri-Chlor tablets has led to a lot of complications for both service pros and pool owners. Liquid sodium hypochlorite and saltwater generators Moving into the 90’s devices began to become more popular. One that increased in a big way was saltwater generators. These were sold to many new pool owners under the auspice of being a “non-chemical” pool that relied only on salt to purify the water. Many of those new pool owners were not aware that the salt unit installed on their new pool was basically a small home version of a largescale liquid sodium hypochlorite factory. Liquid sodium hypochlorite is made at chemical plants by first using a process of electrolysis to split the sodium chloride molecule. Simple salt is divided into chlorine and sodium hydroxide then this is blended with good old H2O to form liquid sodium hypochlorite. The saltwater generators on swimming pools do the same thing on a smaller scale. So, they really are more correctly referred to as chlorine generators. Like any type of chlorine sanitized swimming pool, a chlorine generator pool will still need to have a level of CYA to prevent rapid burn out of chlorine from the UV rays of the sun. CYA levels in a salt pool are good at a level of 30 -50ppm. Using the 7.5% ratio that would mean with a CYA of 30 the ppm of FC needed from the chlorine generator would be 2.25 ppm. At times a chlorine generator may need a back up of manually added chlorine to keep the water quality good. This could be a result of heavy swimmer load, equipment failure, or power outage. Liquid sodium hypochlorite is one of the best back up sanitizers for chlorine generator systems. The main reason for this is due to the by-product that comes from the use of sodium hypochlorite. Let’s look at the different types of chlorine and their by-products: · Calcium hypochlorite – by-product left in the water is calcium · Tri-Chlor – by-product left in the water is cyanuric acid CYA · Liquid Sodium Hypochlorite – by-product left in the water is sodium chloride The only by-product left from using liquid sodium hypochlorite to back up a chlorine generator pool is sodium chloride better known as salt. Out of all the types of chlorine, liquid sodium hypochlorite gives a chlorine generator pool what it needs to function properly. Liquid sodium hypochlorite is a great additive to a salt-chlorine generator system because it can help to replace the salt that can be lost from backwashing or splash out of water.
That means that chlorine still needs to be the primary sanitizer in these pools with a residual between 1-4 ppm. CYA levels need to be controlled in these types of systems in order to get the desired residuals to ensure protection from bacteria in the pool. 30-50 ppm of CYA is the recommended level in these systems. At a CYA level of 60, it would take 4.5 ppm of chlorine to inactivate bacteria. This is outside the required EPA rule. Since the main purpose of secondary devices is to allow for complete disinfection with lower amounts of chlorine, the use of Tri-Chlor in these types of systems would not be a good fit. Again, liquid sodium hypochlorite is a preferred choice for these systems because it provides manageable levels of free chlorine without by-products that will reduce the system's effectiveness of UV, ozone, or AOP.
The benefits of a simple liquid chlorine system for service techs and consumers. With all the chatter, research, and articles of late regarding CYA also comes loads of confusion and questions for service techs and pool owners. There undoubtedly will continue to be continuing research and breakthroughs regarding both the benefits and detriments of using CYA and Tri-Clor in swimming pools. Amidst all this, there is still a reliable, safe, and simple way to get chlorine sanitizer with a measurable residual into swimming pools. Liquid sodium hypochlorite has been proven throughout the history of pool chlorination to be one of the most cost-effective, easy, and safest ways to disinfect pool water. Perhaps this is a good time to return to the simple. Benefits of Liquid Sodium Hypochlorite · Affordable · Easy to use · Safe for storage as it is non-flammable and non-combustible · Leaves a measurable residual of free chlorine · Does not contain calcium or cyanuric acid · Very beneficial as a back up to chlorine generator systems · Ideal for use with secondary sanitizing systems like ozone, UV and AOP *This article is intended for pools outside of fire zones Well, What's It Made Of?
Ash from fires that burn lower than 840 degrees Fahrenheit is mostly organic carbon. At a higher temperature, the carbon is burned away and inorganic compounds are left. These include things like calcium, magnesium, and sodium. The fires in Northern California and the current fires in Southern California not only burned forests but also homes and structures. Because the combustion rate is much higher for buildings, the make-up of the ash is much more different. At a very high combustion rate, the ash can contain potassium and calcium oxides which create quicklime. If enough of this ash gets into the pool and then to the filter, it can create a limestone cement coating on the filter media. Ash from homes and structures can also contain toxins such as lead, arsenic, and hexavalent chromium. Most of the ash that lands in pool water is also hydrophobic and repels water. This explains why the ash floats and is so difficult to remove by skimming. Changing the charge of the ash by using a chitosan clarifier or an enzyme can help in the removal of the ash. What Can I Do? Good news. You are already doing most of the steps that can be taken. A regimen of shock, floc, enzyme, phosphate removal, and algae prevention makes for a good remedial treatment. • Remove all larger debris in the pool and/or spa as soon as possible. • Brush all surfaces thoroughly. • Skim smaller material with a pool net. • Make sure filters are clean and in operational order. • Shock the pool to 20 parts per million (ppm) of Free Chlorine or use a quality chlorine-free oxidizer. • Follow immediately with a clarifier to help send small ash material to the filter. • Consider using an enzyme to help break down some of the non-living, organic material that can’t be filtered out. • Follow with a good broad-spectrum algaecide. • Clean filters are as necessary throughout this process. They may need to be backwashed and cleaned frequently during this time.
How Shocking your Pool Works
Shocking your Pool FAQ When is it time to shock your pool?
Yes, even salt pools need a little help once in a while. Saltwater pools utilize a chlorine generator to convert salt into chlorine. You can adjust the generator to increase the level of chlorine produced to counteract higher chlorine demands caused by contaminants. However, even saltwater pools need to be shocked when the generator cannot keep up with a heavy load of contamination. When should you shock your pool? Regular pool maintenance is essential for healthy, efficient, and economical pool operation. For best results, experts recommend shocking your pool when these circumstances occur:
Shocking your pool is a fairly simple process. Before you begin, uncover your pool, skim the pool, vacuum the sediment, and brush the walls, floor and coves. Before adding shock, you’ll want to protect yourself with the appropriate gear; which includes protective goggles, gloves, and work clothes.
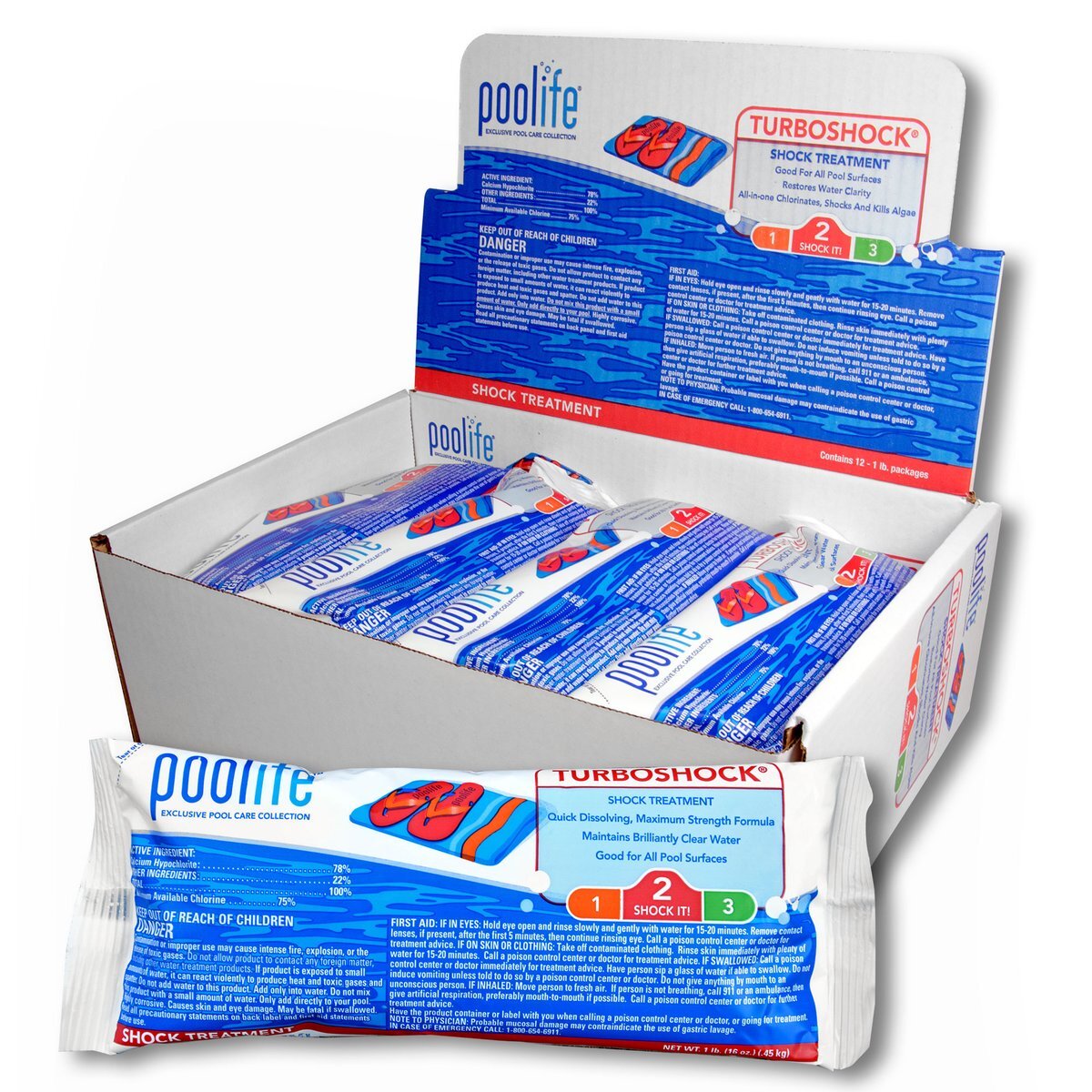
If the free chlorine level is good, a non-chlorine shock or quick shock may be used. These types of shocks allow pool use in as little as 15 minutes. They are very effective at destroying combined chlorine and breaking down contaminants but do not kill algae or raise the free chlorine level. poolife® TurboShock® Shock, 12x1lb. BagsStatistically, August is when the toughest form of MUSTARD ALGAE will appear. Here are a few preventative measures that you can take now.
 How long should I run my pump? It's best to over-filter the water by running the pump about 12 hours per day, give or take, or as needed, to maintain water clarity. Pay close attention to the water quality, or the condition of the water, and you can tell when you need to increase the amount of time that you run the pump each day.
|
Advice, Tutorials & DIY'sAuthorPacific Pool Supply Archives
July 2023
Categories |
|
DO YOU WANT FREE ADVICE AND ACCESS TO EXCLUSIVE DISCOUNTS AND SALES?
SCAN THE QR CODE AND SIGN UP FOR OUR WEEKLY NEWSLETTER! |
STORE HOURS
MON-SAT 9am - 5pm SUNDAY 10am - 3pm 17024 Van Buren Blvd. Ste. D
Riverside, Ca 92504 (in the Stater Bros. Shopping Center) |






































 RSS Feed
RSS Feed

